You wanted an answer, so here it is. Gasoline doesn’t “mysteriously go bad” — it fails for four predictable reasons: oxidation, gum formation, ethanol-water separation, and corrosion. A stabilizer worth the bottle will fight those battles with antioxidant chemistry, water dispersants, and corrosion inhibitors. Everything else you’ll read online? Just noise dressed up as advice.
Students always ask the obvious question first — “what does fuel stabilizer do?” It isn’t magic in a bottle. It slows oxidation, keeps gum precursors from forming, disperses water, and lays down corrosion protection. Simple answer, but the chemistry behind it is what really tells the story.
And then comes the follow-up — “does fuel stabilizer work?” The truth is it depends entirely on how it’s engineered. Built cheap, it won’t. Built right, it absolutely does.
Most people stop right there, skimming for quick fixes and scrolling through lists of ten brands, five brands, sometimes even the same brands rearranged. That’s why they stay stuck repeating the same problems year after year. Nobody explains the chemistry. Nobody shows the tests. And nobody talks about the difference in how these things are actually built.
Because here’s the part you won’t find online. “Not all stabilizers come from the same philosophy. Some are made to scrape by at the lowest possible cost. Some are tuned halfway, a compromise between cost and quality. And a rare few are engineered without compromise — the kind you almost never see on shelves, built by companies that have been at it for decades, quietly setting the real standards the rest of the industry follows years later.”
So if you’re still here, you’ve already set yourself apart. You’re not chasing a shopping list, you’re after understanding. And what follows is the class no one else is teaching — the what, the why, and the how of fuel stability, told with the evidence the crowd ignores.
Gasoline begins degrading the moment it leaves the refinery. Hydrocarbons are unstable molecules, and when stored in the presence of oxygen, heat, and moisture, their structure weakens. Lighter fractions escape through evaporation, leaving behind heavier molecules that bond into longer chains, and the result is gums and varnishes that reduce volatility and choke fuel system passages.
The true enemy in this process is oxidation. Oxygen reacts with hydrocarbons to form unstable peroxides, and once formed, these peroxides set off a chain reaction that accelerates the creation of insoluble gums. Industry measures this vulnerability through the ASTM D525 Oxidation Stability Test, which forces oxygen into heated fuel and records how long it resists peroxide buildup. Untreated gasoline fails quickly, often collapsing within days, while stabilized fuel can extend induction periods by orders of magnitude, transforming weeks of shelf life into many months of usable performance.
Modern pump gasoline is almost never pure hydrocarbons anymore. Nearly all grades contain up to ten percent ethanol, labeled E10, and ethanol brings an entirely new vulnerability into storage. Unlike gasoline itself, ethanol is hygroscopic, meaning it absorbs water molecules directly from the air. Tanks breathe, vent lines admit humidity, and over time that moisture is pulled into the fuel.
Once the threshold is crossed—roughly half of one percent water by volume—the ethanol can no longer hold the load. At that point it separates, dragging water to the bottom of the tank and leaving hydrocarbon gasoline floating above. Engines pull from the lowest point in the tank, so the first liquid into the carburetor or injectors is no longer fuel at all, but a water-rich mixture that causes hard starts, misfires, and sometimes complete failure to ignite.
Phase separation is the silent killer of stored ethanol-blended gasoline. It does not advertise itself until the key is turned and the engine refuses to cooperate. Stabilizers that incorporate polar dispersant chemistry can help suspend trace amounts of water evenly throughout the tank, delaying or preventing the separation. In climates with high humidity, or in marine applications where moisture is a constant threat, this property is not optional—it is the line between fuel that remains viable and fuel that betrays the machine it was meant to run.
Oxidation is the most destructive force acting on stored gasoline. Unlike evaporation, which simply thins the lighter fractions, oxidation rewrites the chemistry of the fuel itself. Hydrocarbons react with oxygen to form unstable peroxides, and those peroxides trigger polymerization into heavier compounds. The result is a sticky film of gums and varnishes that coat every surface fuel touches.
“And here’s where you see the fork in the road. Some stabilizers are built with just enough antioxidant to scrape through a test, and no more — that’s Tier 1 thinking. Others are loaded heavy, pushing oxidation stability into the thousands of minutes. That’s Tier 3. One approach keeps marketing departments happy. The other keeps engines alive. The test doesn’t lie.”
You don’t have to take my word for it — the ASTM D525 test below shows it plain, stopwatch and all. And since I always get asked “how long does fuel stabilizer last?” this is where the answer lives. The induction time in this test tells you exactly how long fuel resists oxidation: hundreds or even thousands of minutes in Tier 3 chemistry versus just a few hundred in Tier 1. That’s the lab proof behind the marketing claims.
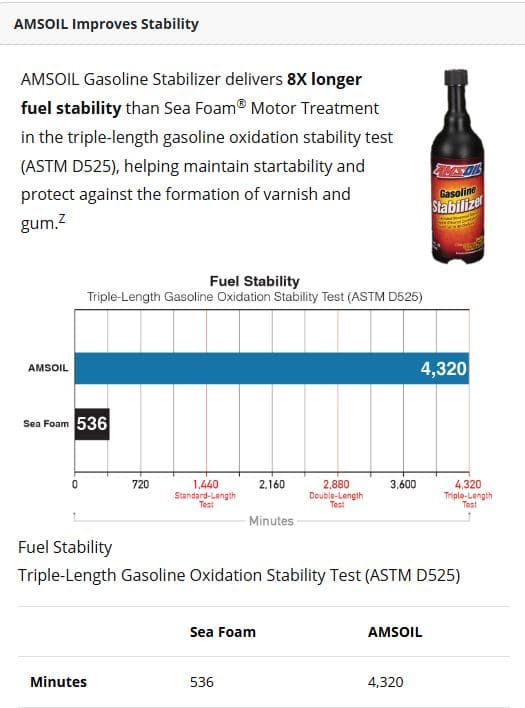
ASTM D525 oxidation stability test. Independent third-party testing compared two common stabilizers under a triple-length procedure. One held stability for the full 4,320 minutes (the test limit), while the other failed at 536 minutes. A longer induction period means fuel resists oxidation, gum formation, and loss of volatility for a significantly extended time.
Source: Independent third-party ASTM D525 testing of AMSOIL Gasoline Stabilizer (July 2022) and Sea Foam Motor Treatment (June 2022). All trademarks are property of their respective owners; no affiliation or endorsement implied.
The industry captures this reality through ASTM D381 gum testing, which makes the problem measurable. Once these deposits take hold, they clog injector tips, adhere to carburetor passages, and glue delicate components like float needles into place. An engine faced with oxidized fuel may refuse to start at all, or worse, run lean and hot until mechanical damage is done. In severe cases, the fuel no longer qualifies as gasoline in a functional sense—it becomes a contaminant.
The measurement that defines this behavior is ASTM D381, the Gum Content test. Fuel is evaporated under controlled heat, and the residue is weighed in milligrams per 100 milliliters. A low number means clean fuel; a high number signals heavy gum loading. It is a direct preview of what will accumulate inside the fuel system after months of dormancy.
“The manuals will tell you gum is just a number on a chart, but out in the shop it’s a death sentence. High residue isn’t theory — it’s the varnish that glues a float needle shut or gums a carb throat until it wheezes. Companies that dose light on antioxidants save pennies and leave you scraping jets with wire. Companies that engineer heavy stop the chain reaction before it starts. That’s the difference between fuel that fires and fuel that fights you.”
Stabilizers built with antioxidant chemistries—commonly amines or phenolics—break this cycle. They intercept free radicals and peroxide intermediates before they can cascade into gums. When properly formulated, these additives can extend the usable life of gasoline from a matter of weeks to a full year, keeping it capable of ignition and preventing the silent destruction that oxidation inflicts.
Stored gasoline is not just unstable in its chemistry; it also turns hostile toward the very hardware meant to contain it. Ethanol blended into modern fuels, along with acidic byproducts of oxidation, combine to attack metal tanks, fuel lines, and carburetor bowls. The corrosion that follows is not cosmetic. Rust, pitting, and etching rob equipment of reliability, weaken components, and cut short the service life of machines that might otherwise endure for decades.
The industry standard that reveals this vulnerability is ASTM D665B, the Rust Prevention Test. In this procedure, a polished steel strip is immersed in treated fuel and then challenged with synthetic seawater to mimic harsh storage conditions. When the strip emerges bright and unblemished, corrosion inhibitors are clearly doing their work. When the strip shows rust, the chemistry has failed, and so too will the equipment over time.
“The ASTM D665B test doesn’t deal in opinions — it shows you steel either protected or eaten alive. A passing strip tells you the inhibitor package is strong enough to stand up to seawater, while a failing one proves the chemistry was thinned out to save cost. Rust in a lab isn’t cosmetic, it’s the same corrosion that chews tanks, pits carb bowls, and seizes fittings when equipment sits. And the thing about rust? It never works part-time. Once it shows up, it’s already on the payroll full-time.”
“This is where compromise shows. Tier 1 products thin the inhibitor package to save a few cents and collapse in the test. Tier 3 loads it heavy, making rust a non-issue even under seawater. The strip doesn’t lie — it tells you how the chemistry was built from the start.”
“And that’s not just theory — you can see it in the following test itself. The strip tells the whole story, bright or rusted, no middle ground.”
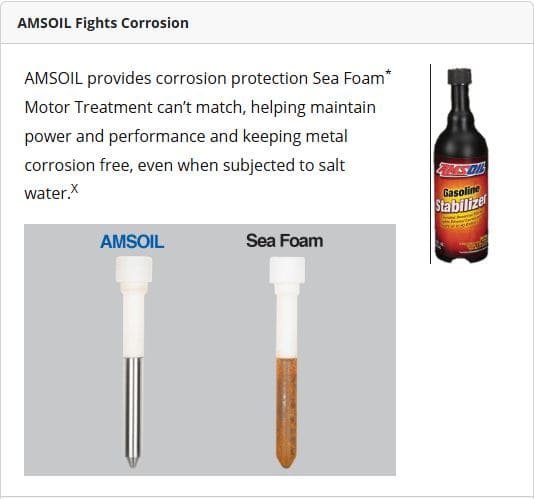
ASTM D665B rust prevention test. In synthetic seawater, a steel strip immersed in treated fuel was evaluated for corrosion. One sample emerged bright and unblemished, while the other showed rust. This illustrates how corrosion inhibitors protect tanks, carburetors, and lines in marine or seasonal storage conditions.
Source: Based on independent third-party testing of AMSOIL Gasoline Stabilizer obtained July 1, 2022, and Sea Foam Motor Treatment purchased June 30, 2022, in a modified NACE TM0172 using synthetic seawater per ASTM D665B. All trademarks are property of their respective owners; no affiliation or endorsement implied.
For marine engines and seasonal storage applications, corrosion protection is not optional—it is essential. Stabilizers that pass this test prove they can hold the line against moisture intrusion and acidic attack. Without that defense, even fresh fuel can leave behind a system scarred with rust and varnish, ensuring that the next start is anything but assured.
And yes, “can fuel stabilizer go bad?” Absolutely. Most formulations have a shelf life of about two years, sometimes less if exposed to heat or air. Like the fuel it protects, stabilizer chemistry doesn’t stay perfect forever — which is why old bottles pulled from a shelf may give you less protection than you expect.
When selecting a fuel stabilizer, the question is not which brand ranks highest on a search page but whether the formulation can withstand the chemistry of time. A stabilizer must show real resistance to oxidation, which industry measures through the ASTM D525 test. This test forces oxygen into heated fuel and times how long it resists peroxide formation, and a product that holds stability in the thousands of minutes rather than hundreds is one that can keep gasoline usable far beyond the untreated norm.
Just as critical is the ability to suppress gum formation, the sticky residue that ASTM D381 quantifies after fuel is evaporated under controlled conditions. A stabilizer that leaves behind minimal residue prevents varnish from coating injectors and carburetor passages, ensuring engines will start and run cleanly even after months of dormancy. This difference in residue can mean the contrast between a quick spring startup and a machine that requires disassembly.
Equally important is the matter of ethanol phase separation. With E10 and E15 fuels absorbing water from the atmosphere, once the threshold of about half a percent by volume is crossed, ethanol and water fall out of solution. A stabilizer that employs polar dispersants to keep that moisture suspended keeps the tank homogeneous and prevents engines from drawing a water-rich layer. Without that protection, phase separation is a silent failure that ruins fuel before the key even turns.
The last measure is corrosion, a threat often underestimated until rust has already pitted the inside of a carburetor bowl or marine tank. ASTM D665B evaluates whether a stabilizer can keep a polished steel strip free of rust even when exposed to synthetic seawater, and a passing result proves that corrosion inhibitors are at work. For seasonal equipment, antique vehicles, and boats, this protection is every bit as essential as oxidation stability itself.
Taken together, these requirements define what makes a stabilizer suitable for storage. For periods of twelve months or more, the chemistry must be rich in antioxidants and corrosion inhibitors that lock fuel in stasis. For shorter spans of six months or less, particularly when carburetors are already dirty, detergents that dissolve varnish and prevent deposits are equally valuable. The best stabilizer is not the one most promoted online, but the one whose formulation aligns with the precise chemical threats of fuel degradation.
The test results tell more than numbers on a chart. They show the philosophy behind the bottle. Tier 1 is the mainstream path—volume first, price point first, engineering last. The chemistry is built just good enough to scrape by, while the real money goes into marketing. No surprise those stabilizers collapse when tested.
Tier 2 makes an effort to do better, but it still lives in compromise. Additive packages are improved, performance is stronger, but the formula is balanced against cost. It works better, but it’s not the whole answer.
Tier 3 is different. These are not the bottles stacked high in every big-box store. Tier 3 stabilizers are built by companies that put engineering ahead of advertising, and they are rarely household names. Some of these manufacturers have been quietly at it for fifty, even a hundred years, moving from strength to strength without ever chasing fads.
They don’t follow trends, they create them. They build products that set the standard years before the rest of the industry catches up. They grow by word of mouth, passed between mechanics, fleet managers, and serious equipment owners who know what works and what doesn’t. That’s why you won’t see them plastered across billboards, but you will see them quietly running in machines that refuse to quit.
And just like you took the time to read this post, if you take the time to seek out the companies building at the Tier 3 level, you’ll find the effort was worth it. The reward comes not in marketing slogans but in results you can feel—in engines that light off clean after storage, in fuel systems free of varnish and corrosion, in equipment that runs the way it was meant to. Performance and protection are never accidents. They are the outcome of engineering choices, and the patience to recognize them.
“So, does fuel stabilizer work? You’ve seen the tests, the chemistry, and the philosophies. The answer is yes — but only when the formulation belongs in Tier 3, not Tier 1.”
For a straightforward overview, AMSOIL’s technical blog has a solid primer on stabilizers here. “Think of it as the 101 course — and what you’ve just read here as the advanced lab.”
